Summary of Dr. Lederer's Research
Excitation-contraction (EC) coupling in heart and the molecular and cellular basis of Ca2+ signaling: the central role of local Ca2+ signaling.
Dr. Lederer, along with an international set of collaborators, have defined the central role of local Ca2+ signaling in excitation-contraction (EC) coupling in heart and the molecular and cellular basis of Ca2+ signaling in excitable cells. Until the discoveries by the Lederer team, there was only modest evidence demonstrating the central role of nanoscopic (molecular, subcellular) Ca2+signaling in EC coupling. Niggli and Lederer 1990 showed that the voltage dependence of the [Ca2+]i signal provides critical information regarding the spatial organization of the EC coupling machinery. Gomez et al. 1997 extended the work to show that in cardiac hypertrophy and heart failure, EC coupling becomes defective. This work took on more significance with the discovery of local Ca2+ signaling and the discovery of Ca2+ sparks and blinks in cardiac, skeletal and smooth muscle.
Local Ca2+ signaling and the discovery of Ca2+ sparks and blinks in cardiac, skeletal and smooth muscle.
The discovery and characterization by the Lederer team (including Drs. Mark Cannell and Heping Cheng) revolutionized our understanding of Ca2+ signaling in cardiac (Cheng et al. 1993), skeletal (Klein et al. 1996) and smooth muscle (Nelson et al. 1995). The study of Ca2+ sparks (Cheng and Lederer 2008) and the reciprocal SR Ca2+ depletion signal, a Ca2+ blink, (Brochet et al. 2005) in heart provided a new mechanistic view of EC coupling, Ca2+ dependent arrhythmogenesis and signaling rules for both the sarcoplasmic reticulum (SR) and other sarcolemma. These Ca2+ release events arise from the coordinated signaling of a cluster of SR Ca2+ release channels (RyRs). Ca2+ release events that could arise from single RyRs (calcium quarks) had been shown to occur in modeling experiments or in vitro in bilayer experiments, but were demonstrated in vivo by the Lederer group (Brochet et al. 2011).
Ca2+ dependent arrhythmogenesis.
Studying normal calcium signaling has led Dr. Lederer and his colleagues to discoveries of the role of calcium in the development of calcium dependent arrhythmias. How Ca2+ signaling dysfunction and SR Ca2+ overload could lead to arrhythmogenesis was first articulated in Lederer and Tsien 1976, with many follow-up papers by Lederer and other investigators. Similar Ca2+ -dependent arrhythmias were shown to arise from mutations in the cytoskeleton (Mohler et al. 2003), mutations in RyR (Wehrens et al. 2003) in heart failure (Song et al. 2006) and in catecholaminergic polymorphic ventricular tachycardia (Lehnart et al. 2008), both in common and rare pathologies.
Chemo-mechanical signaling in muscle: X-ROS signaling in heart and skeletal muscle.
The most recent discoveries link calcium to the contraction machinery. Lederer and his colleagues discovered a new signaling pathway, X-ROS signaling, when studying how mechanical changes in single heart cells can affect Ca2+ signaling (Iribe et al. 2009) with normal physiological and unusual pathological consequences (Prosser et al. 2011). This signaling pathway arises as the mechanical changes in the heart cell shape distort the cellular cytoskeleton (microtubules) which can activate NADPH-oxidase to generate local (subcellular) reactive oxygen species (mainly H2O2). This reactive oxygen reversibly oxidizes diverse targets including RyR and many other targets to increase the rate of SR Ca2+ release and Ca2+ sparks. This is an area of exploding active research in which Lederer and colleagues are very active participants (Khairallah et al. 2012; Ward et al. 2014).
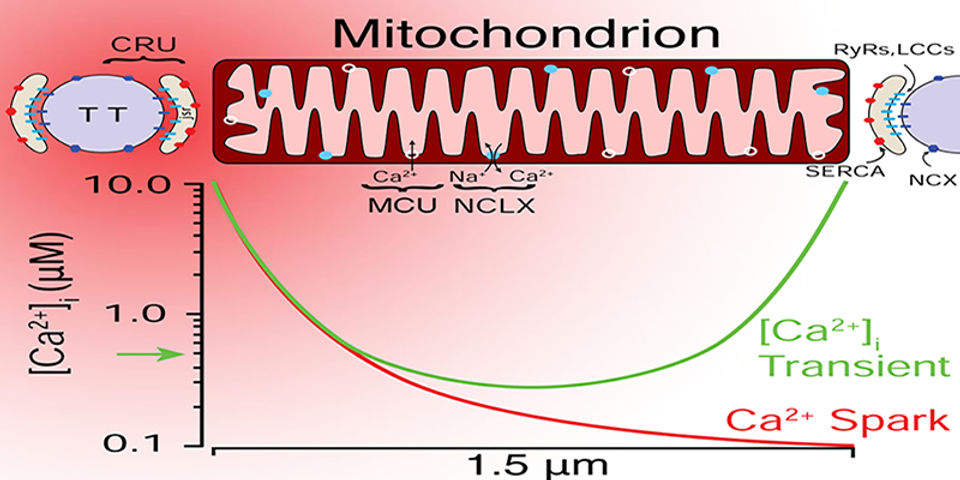
Computational biology: modeling Ca2+ signaling at high temporal and spatial resolution in cardiac myocytes and in cardiac mitochondria.
The most recent work in the Lederer laboratory combines computational biology with high resolution imaging and electrophysiology by modeling Ca2+ signaling at high temporal and spatial resolution in cardiac myocytes and in cardiac mitochondria. When results are organized quantitatively and mechanistically, mathematical modeling or simulations of the experimental findings have rich value. Lederer, his trainees and his colleagues have sought to organize their findings quantitatively as an extension of the experiments. Williams et al. 2011 and Walker et al. 2014 examine Ca2+ sparks, Ca2+ quarks, and [Ca2+]i transients at the cellular and subcellular levels at high temporal and spatial resolution. Boyman et al. 2013, Williams et al. 2013 and Boyman et al. 2014 examine Ca2+ movement in mitochondria.